Selected Publications
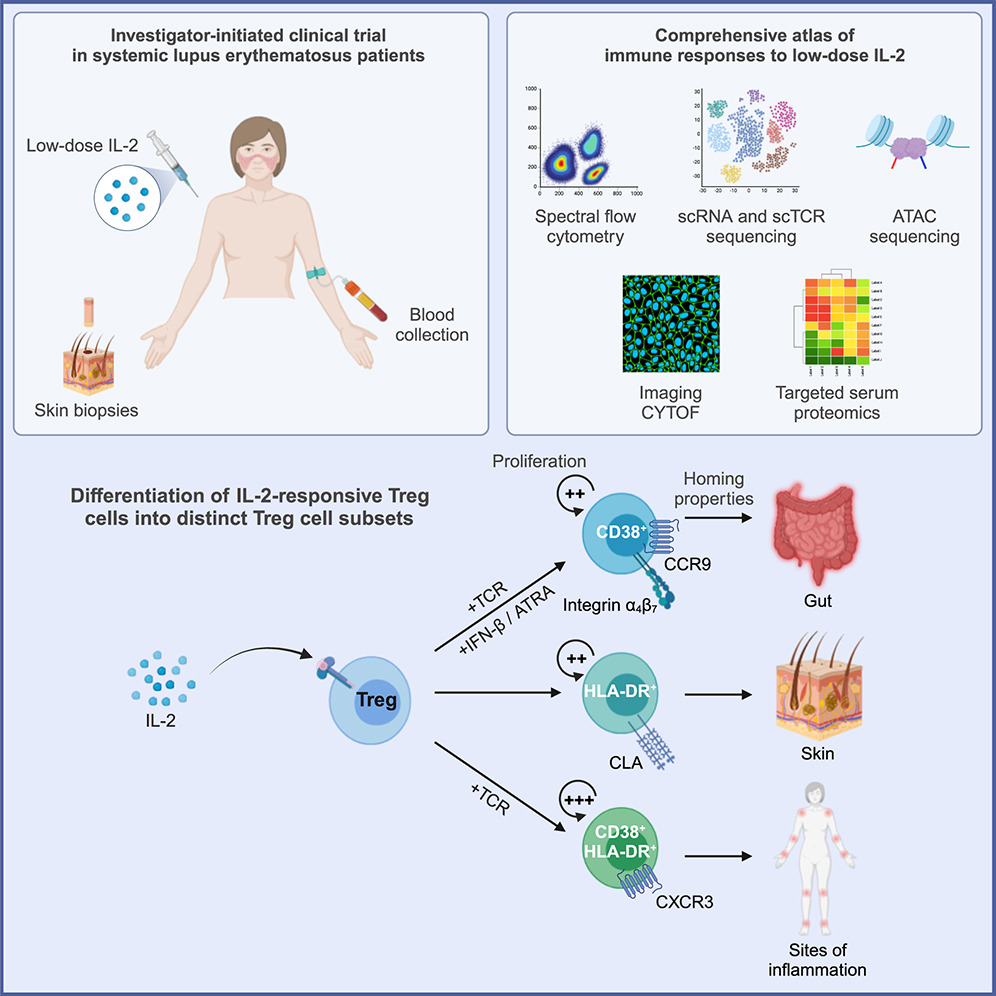
Interleukin-2 immunotherapy reveals human regulatory T cell subsets with distinct functional and tissue-homing characteristics
Raeber ME, Caspar DP, Zurbuchen Y, Guo N, Schmid J, Michler J, Martin AC, Steiner UC, Moor AE, Koning F, Boyman O
Immunity (2024)
DOI: 10.1016/j.immuni.2024.07.016
Abstract
Due to its stimulatory potential for immunomodulatory CD4+ regulatory T (Treg) cells, low-dose interleukin-2 (IL-2) immunotherapy has gained considerable attention for the treatment of autoimmune diseases. In this investigator-initiated single-arm non-placebo-controlled phase-2 clinical trial of low-dose IL-2 immunotherapy in systemic lupus erythematosus (SLE) patients, we generated a comprehensive atlas of in vivo human immune responses to low-dose IL-2. We performed an in-depth study of circulating and cutaneous immune cells by imaging mass cytometry, high-parameter flow cytometry, transcriptomics, and targeted serum proteomics.
Low-dose IL-2 stimulated various circulating immune cells, including Treg cells with a skin-homing phenotype that appeared in the skin of SLE patients in close interaction with endothelial cells. Analysis of surface proteins and transcriptomes revealed different IL-2-driven Treg cell activation programs, including gut-homing CD38+, skin-homing HLA-DR+, and highly proliferative inflammation-homing CD38+ HLA-DR+ Treg cells. Collectively, these data define the distinct human Treg cell subsets that are responsive to IL-2 immunotherapy.

Persistent complement dysregulation with signs of thromboinflammation in active Long Covid
Cervia-Hasler C, Brüningk SC, Hoch T, Fan B, Muzio G, Thompson RC, Ceglarek L, Meledin R, Westermann P, Emmenegger M, Taeschler P, Zurbuchen Y, Pons M, Menges D, Ballouz T, Cervia-Hasler S, Adamo S, Merad M, Charney AW, Puhan M, Brodin P, Nilsson J, Aguzzi A, Raeber ME, Messner CB, Beckmann ND, Borgwardt K, Boyman O
Science (2024)
DOI: 10.1126/science.adg7942
Abstract
Acute infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes various clinical phenotypes, ranging from asymptomatic to life-threatening COVID-19. About 5% of all infected individuals do not recover from acute disease but develop long-term complications, called Long Covid. Current hypotheses on factors contributing to Long Covid include tissue damage, viral reservoirs, autoimmunity, and persistent inflammation. There are currently no diagnostic tests or therapeutic solutions for affected patients.
We followed 39 healthy controls and 113 COVID-19 patients for up to 1 year after initial confirmation of acute SARS-CoV-2 infection to identify biomarkers associated with Long Covid. At 6-month follow-up, 40 patients had Long Covid symptoms. Repeated clinical assessments were paired with blood draws, resulting in a total of 268 longitudinal blood samples. We measured >6500 proteins in serum by proteomics. Top candidate biomarkers were identified using computational tools and further evaluated experimentally.
Our data suggest that active Long Covid is accompanied by a blood protein signature marked by increased complement activation and thromboinflammation, including activated platelets and markers of red blood cell lysis. Tissue injury may also be complement-mediated and, in turn, activate the complement system. Moreover, complement activation may be driven by antigen–antibody complexes, involving autoantibodies and antibodies against herpesviruses, as well as cross-talk with a dysregulated coagulation system. In addition to offering a basis for new diagnostic solutions, our work provides support for clinical research on complement modulators for patients suffering from Long Covid.

Human memory B cells show plasticity and adopt multiple fates upon recall response to SARS-CoV-2
Zurbuchen Y, Michler J, Taeschler P, Adamo S, Cervia C, Raeber ME, Acar IE, Nilsson J, Warnatz K, Soyka MB, Moor AE, Boyman O
Nature Immunology (2023)
DOI: 10.1038/s41590-023-01497-y
Abstract
The B cell response to different pathogens uses tailored effector mechanisms and results in functionally specialized memory B (Bm) cell subsets, including CD21+ resting, CD21–CD27+ activated and CD21–CD27– Bm cells. The interrelatedness between these Bm cell subsets remains unknown. Here we showed that single severe acute respiratory syndrome coronavirus 2-specific Bm cell clones showed plasticity upon antigen rechallenge in previously exposed individuals.
CD21– Bm cells were the predominant subsets during acute infection and early after severe acute respiratory syndrome coronavirus 2-specific immunization. At months 6 and 12 post-infection, CD21+ resting Bm cells were the major Bm cell subset in the circulation and were also detected in peripheral lymphoid organs, where they carried tissue residency markers. Tracking of individual B cell clones by B cell receptor sequencing revealed that previously fated Bm cell clones could redifferentiate upon antigen rechallenge into other Bm cell subsets, including CD21–CD27– Bm cells, demonstrating that single Bm cell clones can adopt functionally different trajectories.

Biased IL-2 signals induce Foxp3-rich pulmonary lymphoid structures and facilitate long-term lung allograft acceptance in mice
Yamada Y, Nguyen T, Impellizzieri D, Mineura K, Shibuya R, Gomariz A, Haberecker M, Nilsson J, Nombela-Arrieta C, Jungraithmayr W, Boyman O
Nature Communications (2023)
DOI: 10.1038/s41467-023-36924-z
Abstract
Insight into the pathomechanism of atopic diseases demonstrated a pivotal role of the cytokines interleukin-4 (IL-4) and IL-13, which has spurred the development of tailored therapeutics targeting their common IL-4 receptor (IL-4R). However, several aspects of the IL-4R system remain ill-defined in humans.
Transplantation of solid organs can be life-saving in patients with end-stage organ failure, however, graft rejection remains a major challenge. In this study, by pre-conditioning with interleukin-2 (IL-2)/anti-IL-2 antibody complex treatment biased toward IL-2 receptor α, we achieved acceptance of fully mismatched orthotopic lung allografts that remained morphologically and functionally intact for more than 90 days in immunocompetent mice. These allografts are tolerated by the actions of forkhead box p3 (Foxp3)+ regulatory T (Treg) cells that home to the lung allografts. Although counts of circulating Treg cells rapidly return to baseline following cessation of IL-2 treatment, Foxp3+ Treg cells persist in peribronchial and peribronchiolar areas of the grafted lungs, forming organized clusters reminiscent of inducible tertiary lymphoid structures (iTLS). These iTLS in lung allografts are made of Foxp3+ Treg cells, conventional T cells, and B cells, as evidenced by using microscopy-based distribution and neighborhood analyses. Foxp3-transgenic mice with inducible and selective deletion of Foxp3+ cells are unable to form iTLS in lung allografts, and these mice acutely reject lung allografts. Collectively, we report that short-term, high-intensity and biased IL-2 pre-conditioning facilitates acceptance of vascularized and ventilated lung allografts without the need of immunosuppression, by inducing Foxp3-controlled iTLS formation within allografts.

Comprehensive analysis of human IL-4 receptor subunits shows compartmentalization in steady state and dupilumab treatment
Heeb L, Boyman O
Allergy (2022)
DOI: 10.1111/all.15576
Abstract
Insight into the pathomechanism of atopic diseases demonstrated a pivotal role of the cytokines interleukin-4 (IL-4) and IL-13, which has spurred the development of tailored therapeutics targeting their common IL-4 receptor (IL-4R). However, several aspects of the IL-4R system remain ill-defined in humans.
We used multicolor spectral flow cytometry to characterize IL-4R subunit expression in 28 human immune cell subsets on protein and mRNA levels and assessed their subcellular distribution by applying a specifically adapted protocol that avoided influence of fixation and permeabilization on fluorochrome and antibody performance. In patients, we investigated possible changes in IL-4Rα distribution before and during treatment with dupilumab, a monoclonal antibody-targeting IL-4Rα.
Whereas all immune cell subsets investigated expressed IL-4Rα and common γ chain protein and mRNA, expression of IL-13Rα1 was restricted to myeloid and B cells. Interestingly, some cells contained considerably more intracellular IL-4R protein than on their surface. Naive B cells were found to carry the highest levels of IL-4Rα distributed evenly between surface and intracellular space, whereas IL-4Rα was found predominantly in intracellular pools in neutrophils. In patients with atopic diseases treated with dupilumab, we observed that engagement of IL-4Rα by dupilumab resulted in internalization of the antibody and decreased total IL-4Rα expression. Notably, even after months of treatment not all intracellular IL-4Rα molecules were occupied by dupilumab, indicating the presence of a “dormant” intracellular IL-4Rα pool that could be mobilized upon certain extrinsic or intrinsic cues.
Collectively, our findings suggest that distinct human immune cell subsets contain surface and intracellular IL-4R pools, which are differently affected by targeted biologic treatment.

Type 2 immune predisposition results in accelerated neutrophil aging causing susceptibility to bacterial infection
Egholm C, Özcan A, Breu D, Boyman O
Science Immunology (2022)
DOI: 10.1126/SciImmunol.ABI9733
Abstract
Atopic individuals show enhanced type 2 immune cell responses and a susceptibility to infections with certain bacteria and viruses. Although patients with allergic diseases harbor normal counts of circulating neutrophils, these cells exert deficient effector functions. However, the underlying mechanism of this dysregulation of neutrophils remains ill defined. Here, we find that development, aging, and elimination of neutrophils are accelerated in mice with a predisposition to type 2 immunity, which, in turn, causes susceptibility to infection with several bacteria. Neutrophil-mediated immunity to bacterial infection was greatly decreased in mice with a genetic or induced bias to type 2 immunity. Abrogation of interleukin-4 (IL-4) receptor signaling in these animals fully restored their antibacterial defense, which largely depended on Ly6G+ neutrophils.
IL-4 signals accelerated the maturation of neutrophils in the bone marrow and caused their rapid release to the circulation and periphery. IL-4–stimulated neutrophils aged more rapidly in the periphery, as evidenced by their phenotypic and functional changes, including their decreased phagocytosis of bacterial particles. Moreover, neutrophils from type 2 immune predisposed mice were eliminated at a higher rate by apoptosis and phagocytosis by macrophages and dendritic cells. Collectively, IL-4 signaling–mediated neutrophil aging constitutes an important adaptive deficiency in type 2 inflammation, contributing to recurrent bacterial infections.

CCR7-guided neutrophil redirection to skin-draining lymph nodes regulates cutaneous inflammation and infection
Özcan A, Collado-Diaz V, Gunzer M, Halin C, Kolios AGA, Boyman O
Science Immunology (2022)
DOI: 10.1126/sciimmunol.abi9126
Abstract
Neutrophils are the first nonresident effector immune cells that migrate to a site of infection or inflammation; however, improper control of neutrophil responses can cause considerable tissue damage. Here, we found that neutrophil responses in inflamed or infected skin were regulated by CCR7-dependent migration and phagocytosis of neutrophils in draining lymph nodes (dLNs). In mouse models of Toll-like receptor–induced skin inflammation and cutaneous Staphylococcus aureus infection, neutrophils migrated from the skin to the dLNs via lymphatic vessels in a CCR7-mediated manner.
In the dLNs, these neutrophils were phagocytosed by lymph node–resident type 1 and type 2 conventional dendritic cells. CCR7 up-regulation on neutrophils was a conserved mechanism across different tissues and was induced by a broad range of microbial stimuli. In the context of cutaneous immune responses, disruption of CCR7 interactions by selective CCR7 deficiency of neutrophils resulted in increased antistaphylococcal immunity and aggravated skin inflammation. Thus, neutrophil homing to and clearance in skin-dLNs affects cutaneous immunity versus pathology.

Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome
Cervia C, Zurbuchen Y, Taeschler P, Ballouz T, Menges D, Hasler S, Adamo S, Raeber ME, Bächli E, Rüdiger A, Stüssi-Helbing M, Huber LC, Nilsson J, Held U, Puhan MA, Boyman O
Nature Communications (2022)
DOI: 10.1038/s41467-021-27797-1
Abstract
Following acute infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) a significant proportion of individuals develop prolonged symptoms, a serious condition termed post-acute coronavirus disease 2019 (COVID-19) syndrome (PACS) or long COVID. Predictors of PACS are needed. In a prospective multicentric cohort study of 215 individuals, we study COVID-19 patients during primary infection and up to one year later, compared to healthy subjects. We discover an immunoglobulin (Ig) signature, based on total IgM and IgG3 levels, which – combined with age, history of asthma bronchiale, and five symptoms during primary infection – is able to predict the risk of PACS independently of timepoint of blood sampling.
We validate the score in an independent cohort of 395 individuals with COVID-19. Our results highlight the benefit of measuring Igs for the early identification of patients at high risk for PACS, which facilitates the study of targeted treatment and pathomechanisms of PACS.

Signature of long-lived memory CD8+ T cells in acute SARS-CoV-2 infection
Adamo S, Michler J, Zurbuchen Y, Cervia C, Taeschler P, Raeber ME, Baghai Sain S, Nilsson J, Moor AE, Boyman O
Nature (2021)
DOI: 10.1038/s41586-021-04280-x
Abstract
Immunological memory is a hallmark of adaptive immunity and facilitates an accelerated and enhanced immune response upon re-infection with the same pathogen. Since the outbreak of the ongoing coronavirus disease 19 (COVID-19) pandemic, a key question has focused on which severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific T cells stimulated during acute infection give rise to long-lived memory T cells. Using spectral flow cytometry combined with cellular indexing of transcriptomes and T cell receptor (TCR) sequencing we longitudinally characterize individual SARS-CoV-2-specific CD8+ T cells of COVID-19 patients from acute infection to one year into recovery and find a distinct signature identifying long-lived memory CD8+ T cells.
SARS-CoV-2-specific memory CD8+ T cells persisting one year after acute infection express CD45RA, interleukin-7 receptor α (CD127), and T cell factor-1 (TCF1), but they maintain low CCR7, thus resembling CD45RA+ effector-memory T (TEMRA) cells. Tracking individual clones of SARS-CoV-2-specific CD8+ T cells, we reveal that an interferon signature marks clones giving rise to long-lived cells, whereas prolonged proliferation and mammalian target of rapamycin (mTOR) signaling are associated with clonal disappearance from the blood. Collectively, we describe a transcriptional signature that marks long-lived, circulating human memory CD8+ T cells following an acute virus infection.

Receptor-gated IL-2 delivery by an anti-human IL-2 antibody activates regulatory T cells in three different species
Karakus U, Sahin D, Mittl PRE, Mooij P, Koopman G, Boyman O.
Science Translational Medicine (2020)
DOI: 10.1126/scitranslmed.abb9283
Abstract
Stimulation of regulatory T (Treg) cells holds great promise for the treatment of autoimmune, chronic inflammatory, and certain metabolic diseases. Recent clinical trials with low-dose interleukin-2 (IL-2) to expand Treg cells led to beneficial results in autoimmunity, but IL-2 immunotherapy can activate both Treg cells and pathogenic T cells.
Use of IL-2 receptor α (IL-2Rα, CD25)–biased IL-2/anti–IL-2 antibody complexes improves [read_more id="1" more="Read more" less="Read less"] IL-2 selectivity for Treg cells; however, the mechanism of action of such IL-2 complexes is incompletely understood, thus hampering their translation into clinical trials. Using a cell-based and dynamic IL-2R platform, we identified a particular anti-human IL-2 antibody, termed UFKA-20. When bound to UFKA-20, IL-2 failed to stimulate cells expressing IL-2Rβ (CD122) and IL-2Rγ (CD132), unless these cells also expressed high amounts of CD25. CD25 allowed IL-2/UFKA-20 complexes to bind, and binding to CD25 in the presence of CD122 and CD132 was followed by rapid dissociation of UFKA-20 from IL-2, delivery of IL-2 to CD122 and CD132, and intracellular signaling. IL-2/UFKA-20 complexes efficiently and preferentially stimulated CD4+ Treg cells in freshly isolated human T cells ex vivo and in mice and rhesus macaques in vivo. The crystal structure of the IL-2/UFKA-20 complex demonstrated that UFKA-20 interfered with IL-2 binding to CD122 and, to a lesser extent, also CD25. Together, we translated CD25-biased IL-2 complexes from mice to nonhuman primates and extended our mechanistic understanding of how CD25-biasing anti-human IL-2 antibodies work, which paves the way to clinical trials of CD25-biased IL-2 complexes.

An IL-2-grafted antibody immunotherapy withpotent efficacy against metastatic cancer
Sahin D, Arenas-Ramirez N, Rath M, Karakus U, Hümbelin M, van Gogh M, Borsig L, Boyman O.
Nature Communications (2020)
DOI: 10.1038/s41467-020-20220-1
Abstract
Modified interleukin-2 (IL-2) formulations are being tested in cancer patients. However, IL-2immunotherapy damages IL-2 receptor (IL-2R)-positive endothelial cells and stimulates IL-2Rα(CD25)-expressing lymphocytes that curtail anti-tumor responses.
A first generation ofIL-2Rβ(CD122)-biased IL-2s addressed some of these drawbacks. Here, we present asecond-generation CD122-biased IL-2, developed by splitting and permanently graftingunmutated human IL-2 (hIL-2) to its antigen-binding groove on the anti-hIL-2 monoclonalantibody NARA1, thereby generating NARA1leukin. In comparison to hIL-2/NARA1 com-plexes, NARA1leukin shows a longer in vivo half-life, completely avoids association withCD25, and more potently stimulates CD8+T and natural killer cells. These effects result instrong anti-tumor responses in various pre-clinical cancer models, whereby NARA1leukinconsistently surpasses the efficacy of hIL-2/NARA1 complexes in controlling metastaticdisease. Collectively, NARA1leukin is a CD122-biased single-molecule construct based onunmutated hIL-2 with potent efficacy against advanced malignancies.
Interleukin-2 signals converge in a lymphoid–dendritic cell pathway that promotes anticancer immunity.
Raeber ME, Rosalia RA, Schmid D, Karakus U, Boyman O.
Science Translational Medicine (2020)
DOI: 10.1126/scitranslmed.aba5464
Abstract
Tumor-infiltrating dendritic cells (DCs) correlate with effective anticancer immunity and improved responsiveness to anti–PD-1 checkpoint immunotherapy. However, the drivers of DC expansion and intratumoral accumulation are ill-defined. We found that interleukin-2 (IL-2) stimulated DC formation through innate and adaptive lymphoid cells in mice and humans, and this increase in DCs improved anticancer immunity. [read_more id="1" more="Read more" less= "Read less"] Administration of IL-2 to humans within a clinical trial and of IL-2 receptor (IL-2R)–biased IL-2 to mice resulted in pronounced expansion of type 1 DCs, including migratory and cross-presenting subsets, and type 2 DCs, although neither DC precursors nor mature DCs had functional IL-2Rs. In mechanistic studies, IL-2 signals stimulated innate lymphoid cells, natural killer cells, and T cells to synthesize the cytokines FLT3L, CSF-2, and TNF. These cytokines redundantly caused DC expansion and activation, which resulted in improved antigen processing and correlated with favorable anticancer responses in mice and patients. Thus, IL-2 immunotherapy–mediated stimulation of DCs contributes to anticancer immunity by rendering tumors more immunogenic. [/read_more]
Media coverage
This publication has been highlighted in several articles for laypersons:
- "Botenstoff Interleukin 2 überwindet die Krebsresistenz" ("The cytokine interleukin 2 overcomes cancer resistance"), Schweizerische Depechenagentur and Science APA (2020), 16 September 2020
- "Forscher umgehen Krebsresistenz" ("Researchers overcome cancer resistance"), 20Minuten (2020), 17 September 2020
- "Boosting immuno-oncology drugs by recruiting immune messenger cells", FierceBiotech (2020), 21 September 2020
- "An unconventional cDC outcome of IL-2 immunotherapy", Accelerating Cancer Immunotherapy Research, summary of the main findings and interview of first author Miro Raeber, ACIR webpage 30 September 2020
- First author Miro Räber explains his main findings in a short research film at JRNL club , April 20, 2021.
Systemic and mucosal antibody secretion specific to SARS-CoV-2 during mild versus severe COVID-19.
Cervia C, Nilsson J, Zurbuchen Y, Valaperti A, Schreiner J, Wolfensberger A, Raeber ME, Adamo S, Emmenegger M, Hasler S, Bosshard PP, De Cecco E, Bächli E, Rudiger A, Stüssi-Helbling M, Huber LC, Zinkernagel AS, Schaer DJ, Aguzzi A, Held U, Probst-Müller E, Rampini SK, Boyman O.
Journal of Allergy and Clinical Immunology (2020)
DOI: 10.1016/j.jaci.2020.10.040
Abstract
BACKGROUND: Infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes an acute illness termed coronavirus disease 2019 (COVID-19). Humoral immune responses likely play an important role in containing SARS-CoV-2, however, the determinants of SARS-CoV-2-specific antibody responses are unclear.
RESULTS: On average, SARS-CoV-2-specific serum IgA titers in mild COVID-19 cases became positive eight days after symptom onset and were often transient, whereas serum IgG levels remained negative or reached positive values 9–10 days after symptom onset. Conversely, patients with severe COVID-19 showed a highly significant increase of SARS-CoV-2-specific serum IgA and IgG titers as a function of duration since symptom onset, independent of patient age and comorbidities. Very high levels of SARS-CoV-2-specific serum IgA correlated with severe acute respiratory distress syndrome (ARDS). Interestingly, some of the SARS-CoV-2-exposed healthcare workers with negative SARS-CoV-2-specific IgA and IgG serum titers had detectable SARS-CoV-2-specific IgA antibodies in their nasal fluids and tears. Moreover, SARS-CoV-2-specific IgA levels in nasal fluids of these healthcare workers were inversely correlated with patient age.
INTERPRETATION: These data show that systemic IgA and IgG production against SARS-CoV-2 develops mainly in severe COVID-19, with very high IgA levels seen in patients with severe ARDS, whereas mild disease may be associated with transient serum titers of SARS-CoV-2-specific antibodies but stimulate mucosal SARS-CoV-2-specific IgA secretion. The findings suggest four grades of antibody responses dependent on COVID-19 severity.
Media coverage
This publication has been highlighted in several articles for laypersons:
- "Wenn der Körper entscheidet, wie schlimm es wird" ("When your body decides how bad it will be"); newspaper article on SARS-CoV-2 antibody tests citing our research article). Der Spiegel (2020), 22 June 2020.
- "Tests weisen nur einen Fünftel nach" ("Tests might be detecting only a fifth of cases"); newspaper article on SARS-CoV-2 antibody tests based on our research article). TagesAnzeiger (2020), 2 June 2020. Replies in Landbote, Thuner Tagblatt etc.
- "Nicht nur im Blut hat es Antikörper" ("Antibodies can be found not only in the blood"); newspaper article on SARS-CoV-2 antibody tests based on our research article). NZZ am Sonntag (2020), 31 May 2020.
- Interview with first author Carlo Cervia on TeleZ (in German, starting 14'04).
Establishment of a scalable microfluidic assay for characterization of population-based neutrophil chemotaxis.
Grigolato F, Egholm C, Impellizzieri D, Arosio P, Boyman O.
Allergy (2020)
DOI: 10.1111/all.14195
Abstract
BACKGROUND: Regulation of neutrophil chemotaxis and activation plays crucial roles in immunity, and dysregulated neutrophil responses can lead to pathology as seen in neutrophilic asthma. Neutrophil recruitment is key for initiating immune defense and inflammation, and its modulation is a promising therapeutic target. Microfluidic technology is an attractive tool for characterization of neutrophil migration. Compared to transwell assays, microfluidic approaches could offer several advantages, including precis e control of defined chemokine gradients in space and time, automated quantitative analysis of chemotaxis, and high throughput.
RESULTS: Our microfluidic device allowed the precise and reproducible determination of the optimal CXCL2 and CXCL8 concentrations for mouse and human neutrophil chemotaxis, respectively. Furthermore, our microfluidic assay was able to measure the equilibrium and real‐time dynamic effects of specific modulators of neutrophil chemotaxis. We demonstrated this concept by showing that IL‐4 receptor signaling in mouse and human neutrophils inhibited their migration toward CXCL2 and CXCL8, respectively, and this inhibition was time‐dependent.
CONCLUSION: Collectively, our microfluidic device shows several advantages over traditional transwell migration assays and its design is amenable to future integration into multiplexed high‐throughput platforms for screening of molecules that modulate the chemotaxis of different immune cells.
Evolution and function of interleukin-4 receptor signaling in adaptive immunity and neutrophils.
Heeb LEM, Egholm C, Boyman O.
Genes and Immunity (2020)
DOI: 10.1038/s41435-020-0095-7
Article PDF
Abstract
The cytokines interleukin (IL)-4 and IL-13, signaling via the IL-4 receptor (IL-4R), orchestrate type 2 immunity to helminth infections and toxins. Activation of epithelial and myeloid cells, and a transient neutrophils influx initiates type 2 immune responses, which are dominated by basophils, eosinophils, mast cells, B cell immunoglobulin E production, and type 2 T helper and T follicular helper cells. Interestingly, IL-4 and IL-13 can curtail chemotaxis and several effector functions of neutrophils in mice and humans.
This inhibitory role of IL-4 and IL-13 probably developed to limit tissue damage by neutrophils during type 2 immunity where a “weep and sweep” response aims at expulsion and decreased fecundity, instead of killing, of macroparasites. Here, we review when IL-4R signaling cytokines appeared during evolution relative to neutrophils and adaptive immunity. Neutrophil-like granular phagocytes were present in invertebrates throughout the bilaterian clade, but we were unable to find data on IL-4, IL-13, or their receptors in invertebrates. Conversely, vertebrates had both adaptive immunity and IL-4, IL-13, and IL-4Rs, suggesting that type 2 cytokines evolved together with adaptive immunity. Further studies are necessary to determine whether IL-4R signaling in neutrophils was established simultaneously with the appearance of adaptive immunity or later.
IL-4 receptor engagement in human neutrophils impairs their migration and extracellular trap formation.
Impellizzieri D, Ridder F, Raeber ME, Egholm C, Woytschak J, Kolios AGA, Legler DF, Boyman O.
Journal of Allergy and Clinical Immunology (2019)
DOI: 10.1016/j.jaci.2019.01.042
Abstract
BACKGROUND: Type 2 immunity serves to resist parasitic helminths, venoms, and toxins, but the role and regulation of neutrophils during type 2 immune responses are controversial. Helminth models suggested a contribution of neutrophils to type 2 immunity, whereas neutrophils are associated with increased disease severity during type 2 inflammatory disorders, such as asthma.
OBJECTIVE: We sought to evaluate the effect of the prototypic type 2 cytokines IL-4 and IL-13 on human neutrophils.
METHODS: Human neutrophils from peripheral blood were assessed without or with IL-4 or IL-13 for (1) expression of IL-4 receptor subunits, (2) neutrophil extracellular trap (NET) formation, (3) migration toward CXCL8 in vitro and in humanized mice, and (4) CXCR1, CXCR2, and CXCR4 expression, as well as (5) in nonallergic versus allergic subjects.
RESULTS: Human neutrophils expressed both types of IL-4 receptors, and their stimulation through IL-4 or IL-13 diminished their ability to form NETs and migrate toward CXCL8 in vitro. Likewise, in vivo chemotaxis in NOD-scid-Il2rg-/- mice was reduced in IL-4-stimulated human neutrophils compared with control values. These effects were accompanied by downregulation of the CXCL8-binding chemokine receptors CXCR1 and CXCR2 on human neutrophils on IL-4 or IL-13 stimulation in vitro. Ex vivo analysis of neutrophils from allergic patients or exposure of neutrophils from nonallergic subjects to allergic donor serum in vitro impaired their NET formation and migration toward CXCL8, thereby mirroring IL-4/IL-13-stimulated neutrophils.
CONCLUSION: IL-4 receptor signaling in human neutrophils affects several neutrophil effector functions, which bears important implications for immunity in type 2 inflammatory disorders.
Endogenous polyclonal anti-IL-1 antibody responses potentiate IL-1 activity during pathogenic inflammation
Spohn, G., Arenas-Ramirez, N., Bouchaud, G., Boyman O.
Journal of Allergy and Clinical Immunology (2017)
DOI: 10.1016/j.jaci.2016.09.033
Abstract
Background: Particular neutralizing mAbs to certain cytokines act as agonists in vivo through protection of the cytokine's active site and prolongation of its half-life. Although this principle might be useful for targeted immunotherapy, its role in the pathogenesis of inflammation and autoimmunity is unclear.
Objective: We sought to determine whether slight, structurally nonrelevant modifications of the prototypic proinflammatory cytokine IL-1β during an immune response could elicit polyclonal anti-IL-1β antibody responses that modulated IL-1β's in vivo activity.
Methods: We engineered 2 different IL-1β variants, thereby mimicking the process of cytokine modification occurring during inflammation, and conjugated them to virus-like particles, followed by immunization of mice. The resulting polyclonal anti-IL-1β antibody responses were assessed by using in vitro and in vivo assays, as well as 2 relevant (auto-) inflammatory murine models.
Results: Although antibody responses generated to one variant were potently inhibiting IL-1β, antibody responses induced by the other variant even potentiated the in vivo effects of IL-1β; the latter led to enhanced morbidity in 2 different IL-1β-mediated mouse models, including a model of inflammatory bowel disease and an inflammatory arthritis model.
Conclusion: These data demonstrate that endogenous polyclonal anti-cytokine antibody responses can enhance the cytokine's activity in inflammatory and autoimmune diseases.
The Histone Methyltransferase Ezh2 Controls Mechanisms of Adaptive Resistance to Tumor Immunotherapy
Zingg D, Arenas-Ramirez N, Sahin D, Rosalia RA, Antunes AT, Haeusel J, Sommer L, Boyman O.
Cell Reports (2017)
DOI: 10.1016/j.celrep.2017.07.007
Abstract
Immunotherapy and particularly immune checkpoint inhibitors have resulted in remarkable clinical responses in patients with immunogenic tumors, although most cancers develop resistance to immunotherapy. The molecular mechanisms of tumor resistance to immunotherapy remain poorly understood.
We now show that induction of the histone methyltransferase Ezh2 controls several tumor cell-intrinsic and extrinsic resistance mechanisms. Notably, T cell infiltration selectively correlated with high EZH2-PRC2 complex activity in human skin cutaneous melanoma. During anti-CTLA-4 or IL-2 immunotherapy in mice, intratumoral tumor necrosis factor-α (TNF-α) production and T cell accumulation resulted in increased Ezh2 expression in melanoma cells, which in turn silenced their own immunogenicity and antigen presentation. Ezh2 inactivation reversed this resistance and synergized with anti-CTLA-4 and IL-2 immunotherapy to suppress melanoma growth. These anti-tumor effects depended on intratumorally accumulating interferon-γ (IFN-γ)-producing PD-1low CD8+ T cells and PD-L1 downregulation on melanoma cells. Hence, Ezh2 serves as a molecular switch controlling melanoma escape during T cell-targeting immunotherapies.
Improved cancer immunotherapy by a CD25-mimobody conferring selectivity to human interleukin-2
Arenas-Ramirez N, Zou C, Popp S, Zingg D, Brannetti B, Wirth E, Calzascia T, Kovarik J, Sommer L, Zenke G, Woytschak J, Regnier CH, Katopodis A, Boyman O.
Science Translational Medicine (2016)
DOI: 10.1126/scitranslmed.aag3187
Abstract
Interleukin-2 (IL-2) immunotherapy is an attractive approach in treating advanced cancer. However, by binding to its IL-2 receptor α (CD25) subunit, IL-2 exerts unwanted effects, including stimulation of immunosuppressive regulatory T cells (Tregs) and contribution to vascular leak syndrome.
We used a rational approach to develop a monoclonal antibody to human IL-2, termed NARA1, which acts as a high-affinity CD25 mimic, thereby minimizing association of IL-2 with CD25. The structure of the IL-2–NARA1 complex revealed that NARA1 occupies the CD25 epitope of IL-2 and precisely overlaps with CD25. Association of NARA1 with IL-2 occurs with 10-fold higher affinity compared to CD25 and forms IL-2/NARA1 complexes, which, in vivo, preferentially stimulate CD8+ T cells while disfavoring CD25+ Tregs and improving the benefit–to–adverse effect ratio of IL-2. In two transplantable and one spontaneous metastatic melanoma model, IL-2/NARA1 complex immunotherapy resulted in efficient expansion of tumor-specific and polyclonal CD8+ T cells. These CD8+ T cells showed robust interferon-γ production and expressed low levels of exhaustion markers programmed cell death protein-1, lymphocyte activation gene-3, and T cell immunoglobulin and mucin domain-3. These effects resulted in potent anticancer immune responses and prolonged survival in the tumor models. Collectively, our data demonstrate that NARA1 acts as a CD25-mimobody that confers selectivity and increased potency to IL-2 and warrant further assessment of NARA1 as a therapeutic.
Type 2 Interleukin-4 Receptor Signaling in Neutrophils Antagonizes Their Expansion and Migration during Infection and Inflammation
Woytschak J, Keller N, Krieg C, Impellizzieri D, Thompson RW, Wynn TA, Zinkernagel AS, Boyman O.
Immunity (2016)
DOI: 10.1016/j.immuni.2016.06.025
Abstract
Neutrophils are the first immune cells recruited to sites of inflammation and infection. However, patients with allergic disorders such as atopic dermatitis show a paucity of skin neutrophils and are prone to bacterial skin infections, suggesting that allergic inflammation curtails neutrophil responses.
Here we have shown that the type 2 cell signature cytokine interleukin-4 (IL-4) hampers neutrophil expansion and migration by antagonizing granulocyte colony-stimulating factor (G-CSF) and chemokine receptor-mediated signals. Cutaneous bacterial infection in mice was exacerbated by IL-4 signaling and improved with IL-4 inhibition, each outcome inversely correlating with neutrophil migration to skin. Likewise, systemic bacterial infection was worsened by heightened IL-4 activity, with IL-4 restricting G-CSF-induced neutrophil expansion and migration to tissues by affecting CXCR2-CXCR4 chemokine signaling in neutrophils. These effects were dependent on IL-4 acting through type 2 IL-4 receptors on neutrophils. Thus, targeting IL-4 might be beneficial in neutropenic conditions with increased susceptibility to bacterial infections.
Full list of publications:
Book Chapters
Interleukin 2
Natalia Arenas-Ramirez, Boyman O.
Encyclopedia of Inflammatory Diseases (Springer). 2015 June 11, pp. 1-9. DOI: 10.1007/978-3-0348-0620-6_132-1
The Role of Interleukin-2 in Memory CD8 Cell Differentiation
Boyman O., Cho JH., Sprent J.
In: Zanetti M., Schoenberger S.P. (eds) Memory T Cells. Advances in Experimental Medicine and Biology, vol 684. Springer, New York, NY DOI: 10.1007/978-1-4419-6451-9_3










